Abstract
Background: Extranodal Natural Killer/T Cell Lymphoma (ENKTL) is an aggressive non-Hodgkin lymphoma with a high incidence in Asian and south American populations. Pegaspargase, oxaliplatin plus gemcitabine are considered as the standard first-line therapy for advanced ENKTL, but the prognosis remains poor. In ENKTL, most patients overexpress PD-L1 protein, which is associated with a poor prognosis, and suggests that ENKTL should be sensitive to PD-1/PD-L1 axis blockade. In a phase II trial, the PD-1 mAb sintilimab showed promising efficacy as monotherapy in r/r ENKTL (Tao, Signal Transduct Target Ther, 2022). Additionally, we previously conducted a retrospective study of PD-1 mAb combined P-GEMOX for the treatment of advanced ENKTL patients (pts), which showing favorable antitumor activity with a non-overlapping toxicity profile (Cai, Signal Transduct Target Ther, 2020). Therefore, we conducted this prospective phase II trial to evaluate the efficacy and safety of sintilimab plus P-GEMOX as first-line therapy in pts with advanced ENKTL.
Methods: This is a prospective, single-arm, open-label, multi-center, phase II clinical trial. Adult treatment-naive pts with histologically confirmed ENKTL nasal type had at least one measurable or evaluable lesion were enrolled to receive six cycles of sintilimab 200 mg plus pegaspargase 2000 U/m2 (day 1), gemcitabine 1g/m2 (days 1 and 8), and oxaliplatin 130 mg/m2 (day 1) (induction phase), followed by sintilimab 200 mg maintenance every 3 weeks (maintenance phase) for up to 2 years or until disease progression or unacceptable toxicities. Involved field radiotherapy (IFRT) is allowed for residual lesions after induction therapy. The primary endpoint was the proportion of pts who achieved complete response rate (CR) in response evaluable population. Secondary endpoints included overall response rate (ORR), response at the end of induction therapy (EoT), duration of response (DOR), progression-free survival (PFS), overall survival (OS), disease control rate (DCR), and safety. Treatment related adverse events (TRAE) were rated according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) 5.0. This trial is registered with ClinicalTrials.gov, number NCT04127227.
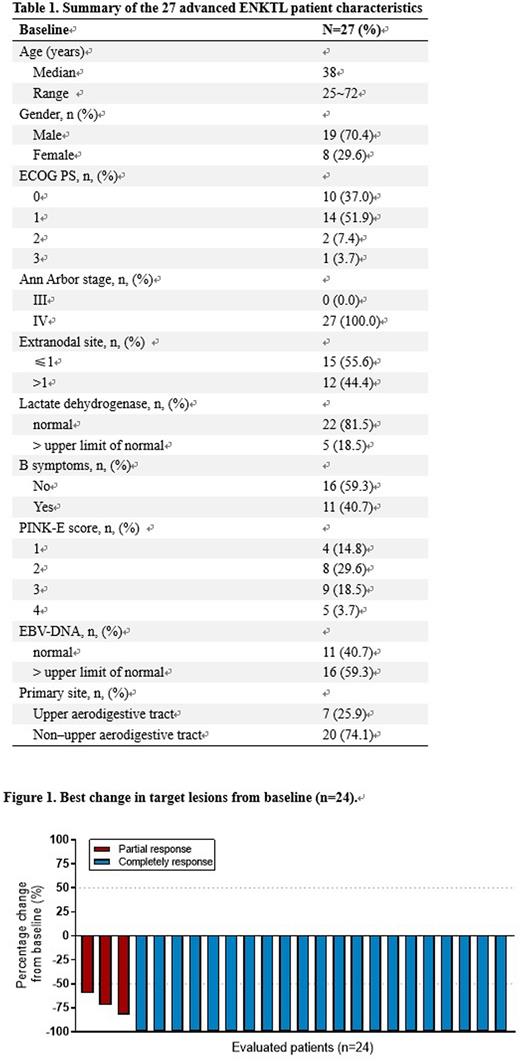
Results: From November 2019 to April 2022, 27 eligible pts were enrolled. Median age was 38 years (range, 25-72), 19 (70.4%) were male, 14 (22.2%) pts had PINK-E score ≥ 3 points (Table 1). Of 24 response evaluable pts, the ORR was 100% (24/24), and the CR rate reached 87.5% (21/24, figure 1, 2). At data cut-off, 18 pts completed 6 cycles of induction therapy and entered the maintenance phase, 1 patient experienced disease progression after 4 cycles of the treatment, and the EoT CR rate of induction phase is 13/19 (68.4%). The median time to response (TTR) was 1.6 (1.4-3.8) months. During the maintenance phase, 5 more pts achieved CR and at the time of analysis, The median follow-up time was 16.7 (3.0-32.0) months. The mOS and mPFS were not reached, estimated 1-year OS rate was 100%, 1-year PFS rate was 95%.All (100%) pts experienced TRAE at any grade. The most common toxicity was neutropenia (96.3%) and the majority of TRAEs were reported during the first two cycles of the induction therapy. Grade 3-4 TRAE reported in ≥5% of pts included neutropenia (48.1%), triglycerides increased (37.0%), leukopenia (29.5%), anemia (25.9%), thrombocytopenia (25.9%), albumin decreased (7.4%), and lipase increased (7.4%). Immune-related AEs (irAEs) were reported in 16 pts, including a grade 3 hypothyroidism which led to permanent treatment discontinuation in 1 (3.7%) patient, and the remaining irAEs were all grade 1-2. All TRAEs were manageable and only 1 patient discontinued treatment due to TRAEs during maintenance phase.
Conclusions: Combination of sintilimab plus P-GEMOX Regimen yielded promising clinical activity with a manageable safety profile, supporting immunochemotherapy as a new first-line treatment option for pts with advanced ENKTL. Treatment and follow-up are currently ongoing and further studies have been initiated to explore biomarkers to better predict response and long-term survival.
Disclosures
No relevant conflicts of interest to declare.
OffLabel Disclosure:
Sintilimab (Tyvyt) is a fully human IgG4 monoclonal antibody that binds to programmed cell death receptor-1 (PD-1). Sintilimab mono/combination therapy has been approved for several indications in mainland of China: Monotherapy for more than 2 lines classical Hodgkin's lymphoma Combination with chemotherapy for the first line treatment of EGFR/ALK negative NSCLC Combination with bevacizumab for the first line treatment of HCC Combination with chemotherapy for the first line treatment of ESCC Combination with chemotherapy for the first line treatment of GC/GEJ The purpose of sintilimab applied in advanced ENKTL is presented in abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal